Heterogeneous disease
Parkinson’s manifests across dopaminergic, cholinergic, cognitive, and motor domains. Capturing heterogeneity demands multimodal evidence and robust harmonization.
Progressive AI harmonizes imaging, diffusion, biospecimen, wearable, and clinical data into interpretable latent spaces and clinician-facing tools. Four domain thrusts build high-quality modality pipelines, while two workflows transform those signals into decision-ready intelligence.
This page details how the program is structured, the workflows we operate, and how thrusts and workflows reinforce one another.
Parkinson’s manifests across dopaminergic, cholinergic, cognitive, and motor domains. Capturing heterogeneity demands multimodal evidence and robust harmonization.
Clinic visits miss day-to-day variation. Imaging, diffusion, and wearables fill the gaps and provide quantitative anchors for precision interventions.
Insights must reach neurologists with context. Our workflows produce interpretable, auditable outputs that connect data science to bedside action.
Modality-focused teams deliver reliable feature extraction for imaging, diffusion, wearable-cognitive phenotyping, and mechanism inference. Each thrust enforces rigorous quality control, provenance tracking, and quantitative validation.
Visit the Domain Thrusts page for full briefs, figures, and cohort specifics.
Workflow 1 builds generative latent spaces that align modalities; Workflow 2 delivers interpreted insight to clinicians via ActionIntel, Free Motion, and sensor-clinical correlation dashboards.
Explore both on the Workflows page.
Harmonizes imaging, diffusion, and clinical data into interpretable latent spaces that seed state estimators and policy agents.
Delivers multimodal inferences to neurologists via interactive visualization, case review, and cohort monitoring tools.
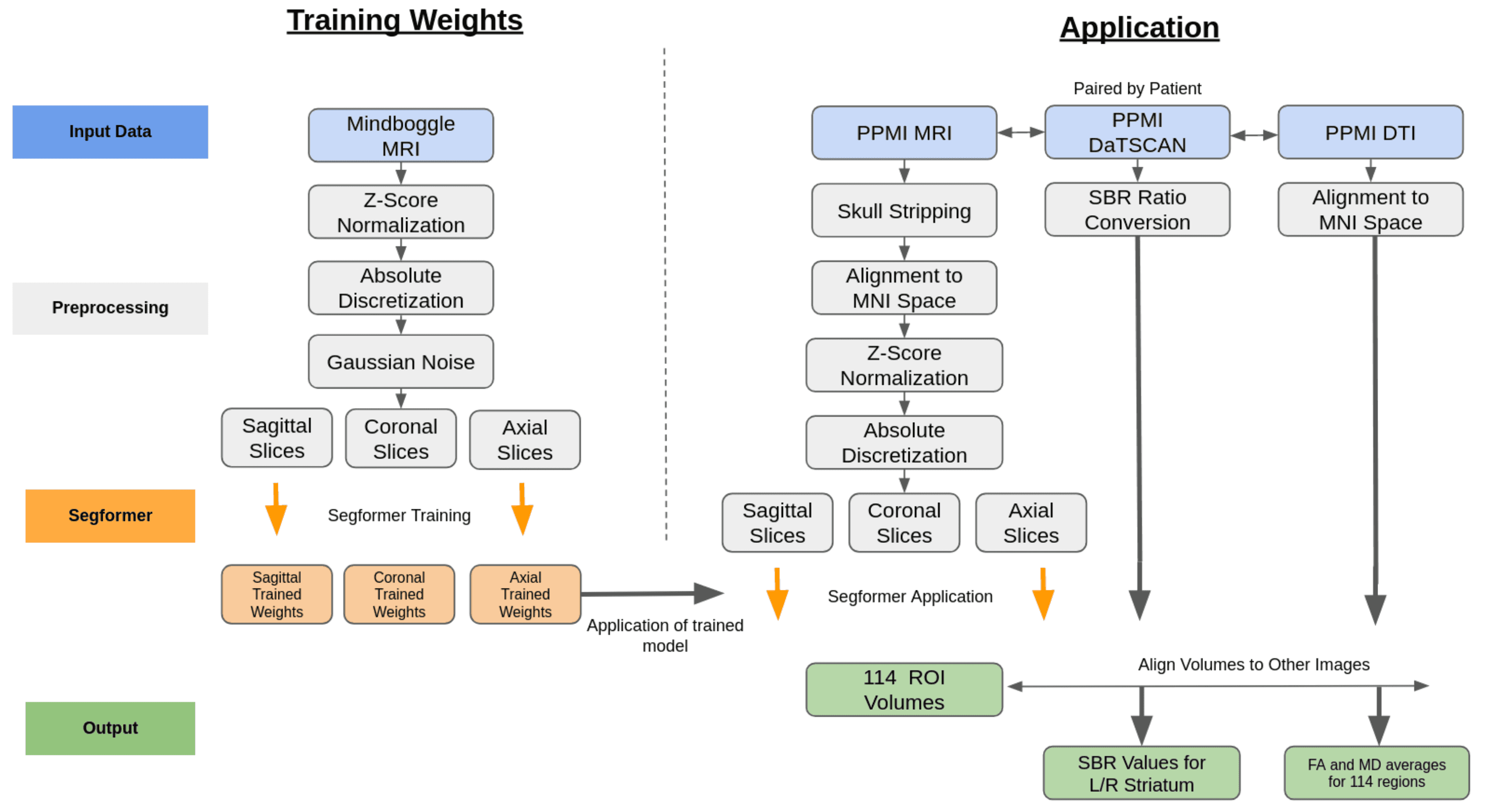
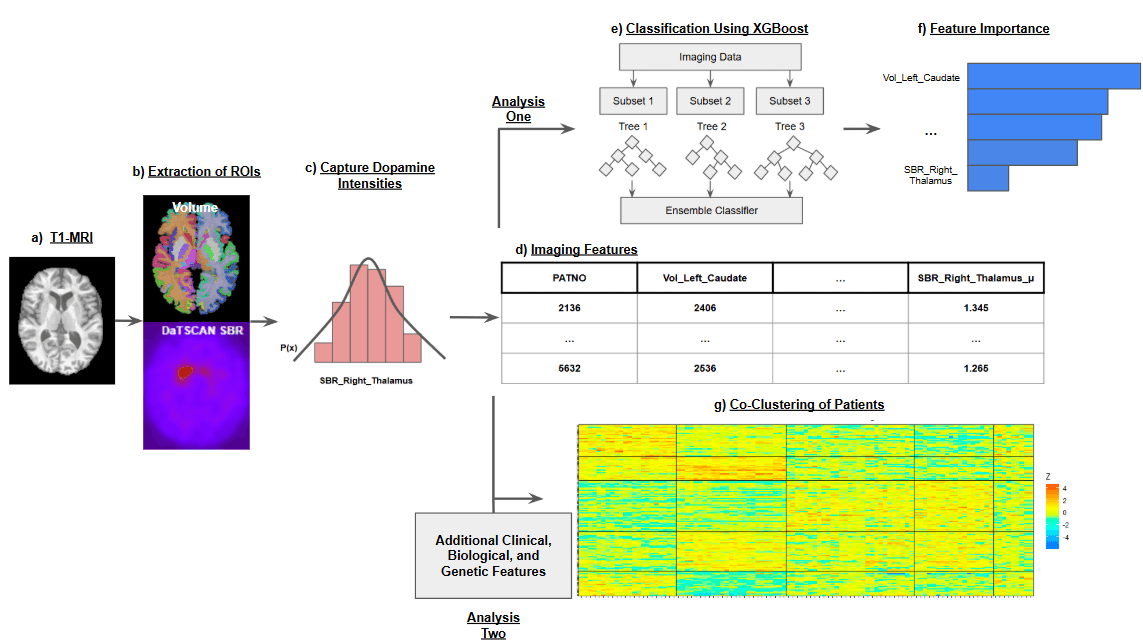
Automated DaT-SPECT and T1 MRI pipelines generate harmonized biomarker matrices that sharpen CSFSAA stratification and feed downstream agents.
Free-water–corrected diffusion metrics align with clinical scales inside a compositional Bayesian co-clustering framework, revealing severity-aligned patient subtypes.
Wearable IMU data, cognitive batteries, and demographics combine to predict CSFSAA status and uncover multimodal phenotypes.
Large-scale statistical testing links multimodal biomarkers to putative biological pathways, grounding discoveries in mechanism-centric hypotheses.
Read the detailed thrust briefs, walk through each workflow, or explore background context on Parkinson’s neurobiology. Complementary resources live on the Thrusts, Workflows, and Background pages.